The smallest bit of matter you can have and have it still be distinct from other bits of matter is an atom. The ingredients of one atom—protons, neutrons and electrons—look just like the ingredients of another atom; that is, all protons look the same as each other and all neutrons look the same as each other, and so on. The reason atoms don’t look the same as each other is because each atom has different number of these ingredients—different numbers of protons, neutrons and electrons.
Each distinct type of atom is called an element. Due to their different numbers of protons, neutrons and electrons, each element has a specific structure and a specific energy signature. The element this cart is mainly concerned with is carbon. There is carbon in every living thing on Earth; every living thing has DNA and DNA is based on carbon. Carbon can also be found in non-living things such as rocks and air.
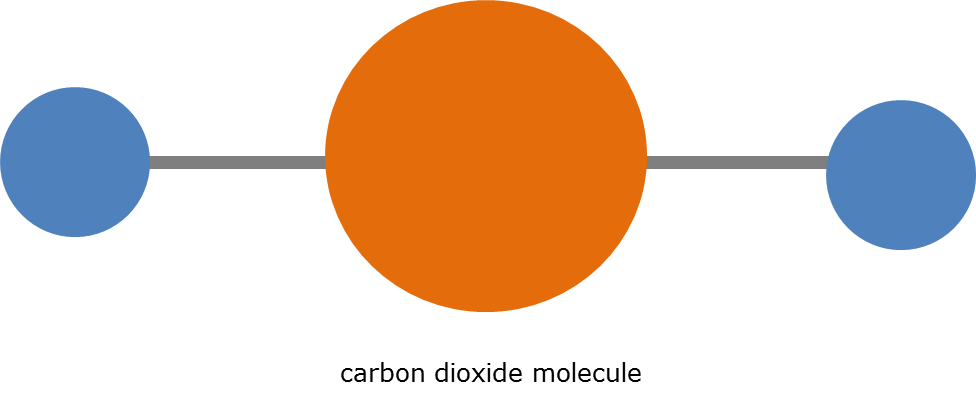 DNA is a molecule. A molecule is formed when atoms bond together. DNA is an extremely complex molecule because it has several elements and many atoms and many bonds, but this cart is mainly concerned with a fairly simple molecule known as carbon dioxide. Carbon dioxide is formed from two oxygen atoms and one carbon atom. Carbon dioxide also has a specific structure and specific energy signature due to its atoms.
DNA is a molecule. A molecule is formed when atoms bond together. DNA is an extremely complex molecule because it has several elements and many atoms and many bonds, but this cart is mainly concerned with a fairly simple molecule known as carbon dioxide. Carbon dioxide is formed from two oxygen atoms and one carbon atom. Carbon dioxide also has a specific structure and specific energy signature due to its atoms. Carbon dioxide is sometimes called CO2; the small “2” denotes that there are two oxygen atoms.
Next
General background information: Carbon | Climate Change | Communication Traps | Climate Change Solutions | Climate Change Pitfalls