Electromagnetic radiation is a kind of radiation. The electromagnetic radiation we can see is light.
Electromagnetic radiation has properties of both particles and waves. The particle form of electromagnetic radiation is a photon. You can think of photons as tiny chunks of energy. Photons have no mass and don’t take up space, and yet we think of them as particles because electromagnetic radiation travels in little bursts, not a smooth uniform wave.
For now, you can think of electromagnetic radiation as a wave. To do this, imagine electromagnetic radiation as a line of energy coming toward you, moving at the speed of light. That wave is oscillating, meaning the line is moving up and down, like this:
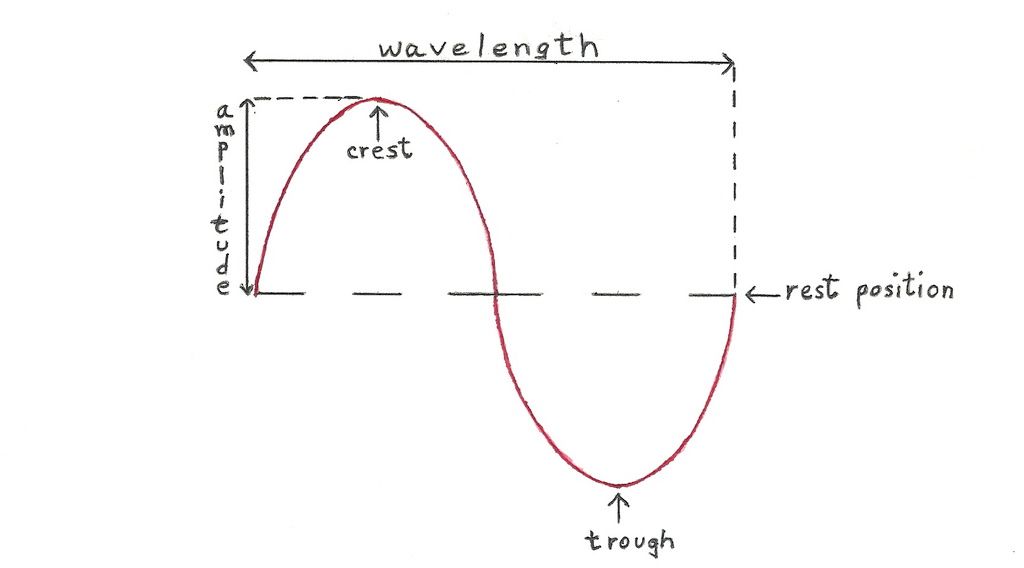
For an electromagnetic wave, the amplitude will tell you the intensity of the light. The frequency will tell you the energy of the light.
Background Information for Activity #2: Spectroscopy: Electromagnetic Radiation | Electromagnetic Frequency | Light | Generating Electromagnetic Radiation | Sources of Electromagnetic Radiation | Reflection and Absorption | Atomic Spectroscopy | Molecular Spectrscopy